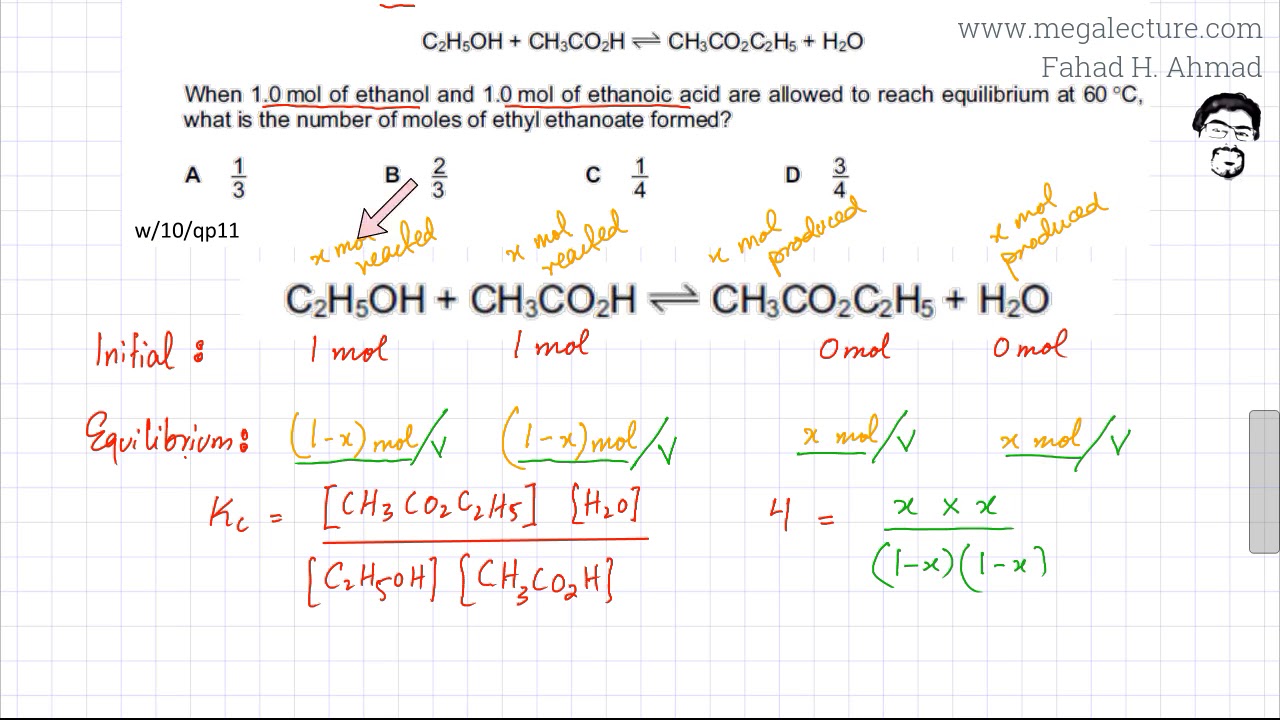
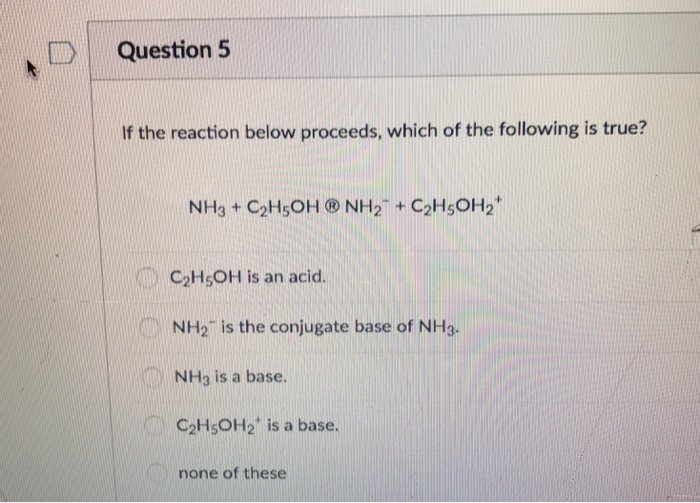
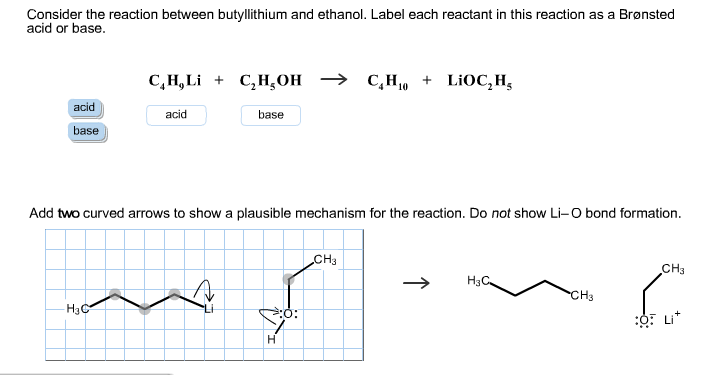
SOLVED: C2H5OH is an substance and a a. organi, non, electrolyte b. organic, strong electrolyte c. strong base, strong electrolyte d. strong acid, strong electrolyte

Growth Pattern, Stability, and Properties of Complexes of C2H5OH and nCO2 (n = 1–5) Molecules: A Theoretical Study | ACS Omega
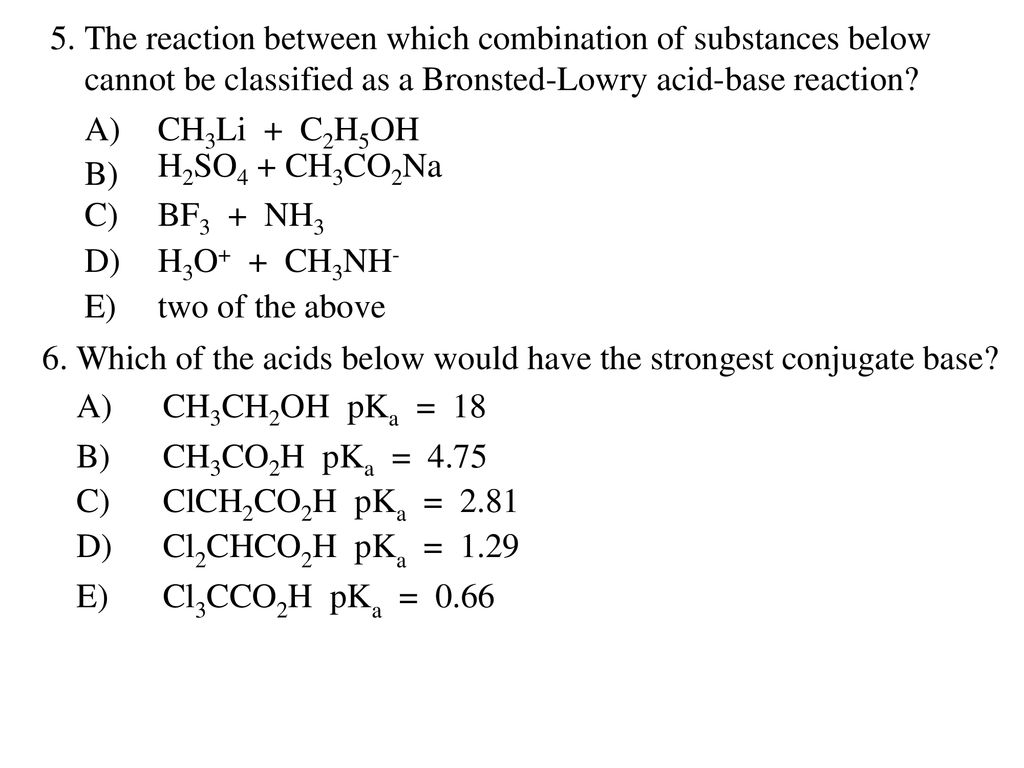
1. Which of the following is not both a Bronsted-Lowry acid and a Bronsted-Lowry base? A) HSO4- (hydrogen sulfate) B) H2PO4- (dihydrogen phosphate) - ppt download

SOLVED: Identify each substance as an Arrhenius acid, an Arrhenius base, or neither. NaOH C2H5OH H3PO4

Reagents and conditions: (a) CCl3CH(OH)2, NH2OH. HCl, 45min heating;... | Download Scientific Diagram
Which of the following reacts is not shown by formic acid? Reaction with Ca(OH)2 Reaction with I2 / Red P Reaction with NaHCO3 Reaction with C2H5OH
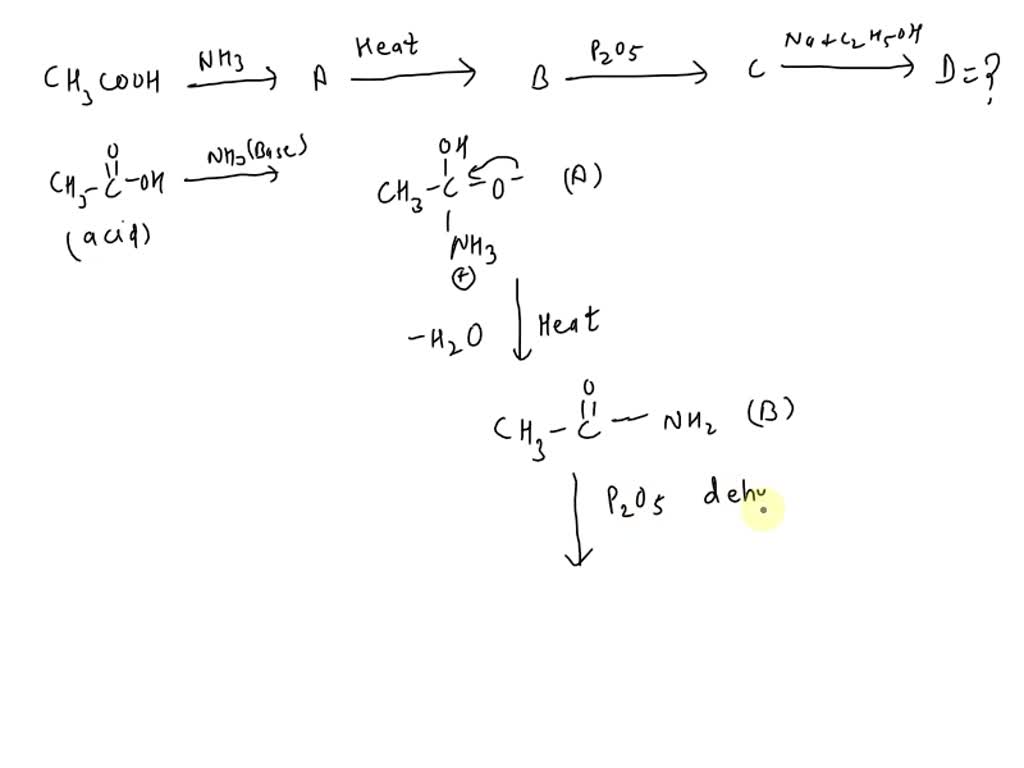
SOLVED: The product (D) in the following sequence of reactions is: CH3COOH NH3 (A) Heat (B) P2O5 (C) Na + C2H5OH (D) A. ester B. amine C. acid D. alcohol