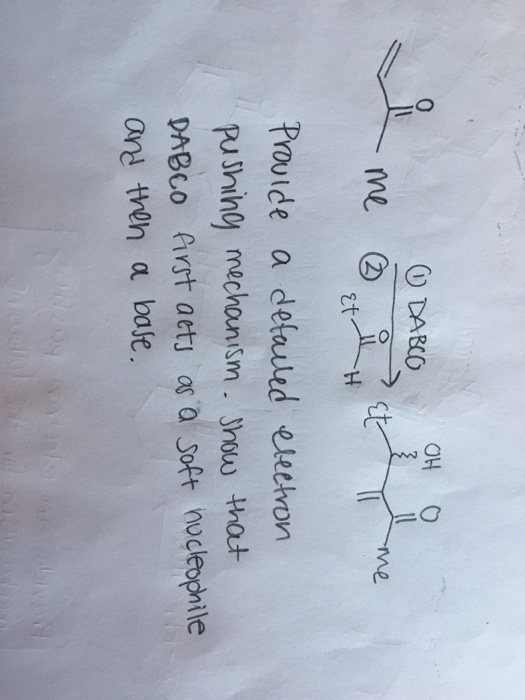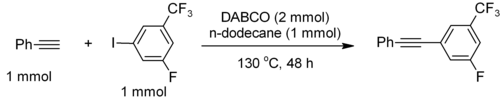Lewis Base‐Brønsted Acid Co‐catalyzed Morita‐Baylis‐Hillman Reaction of Cyclic Sulfamidate Imines - Khassenova - 2021 - European Journal of Organic Chemistry - Wiley Online Library
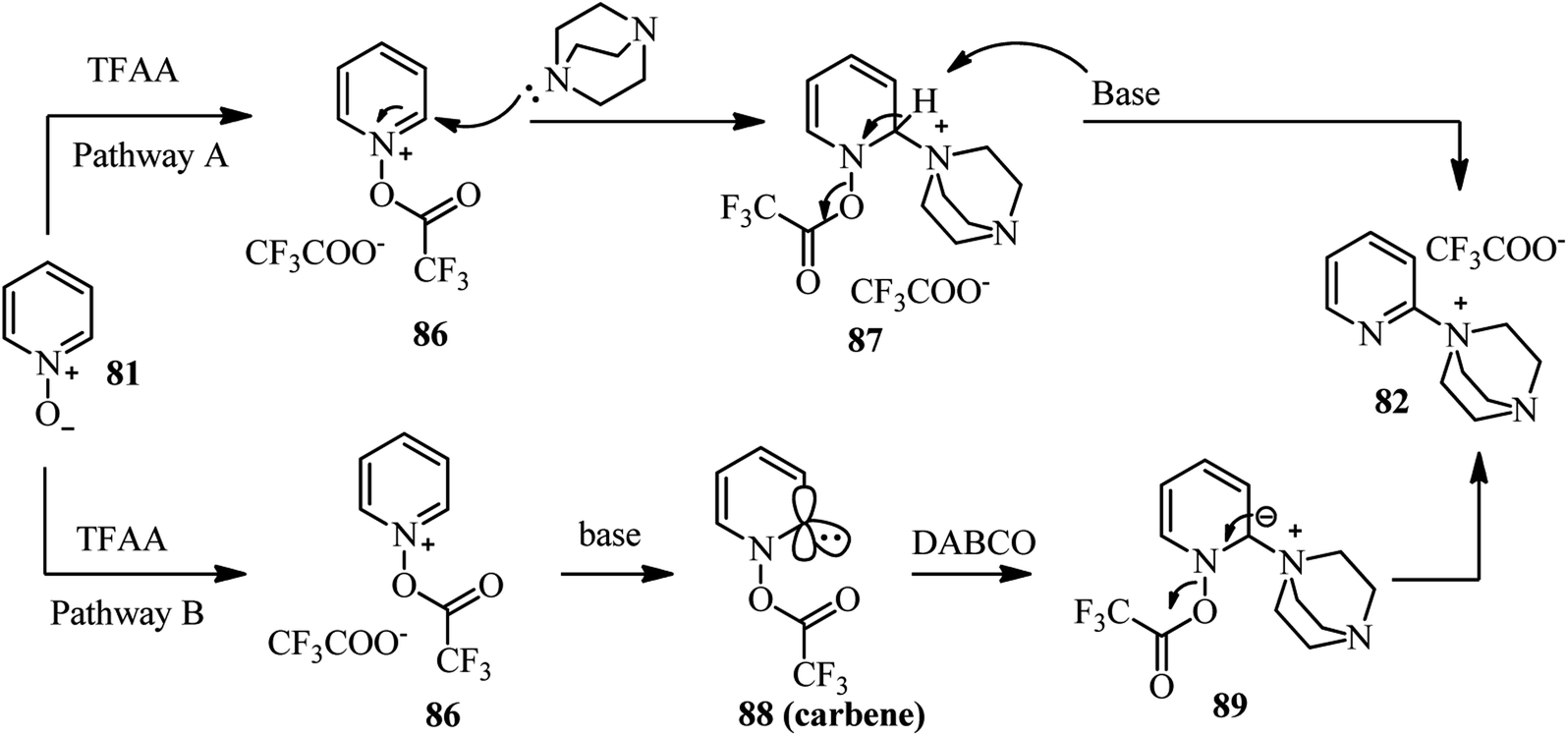
DABCO bond cleavage for the synthesis of piperazine derivatives - RSC Advances (RSC Publishing) DOI:10.1039/C9RA07870C
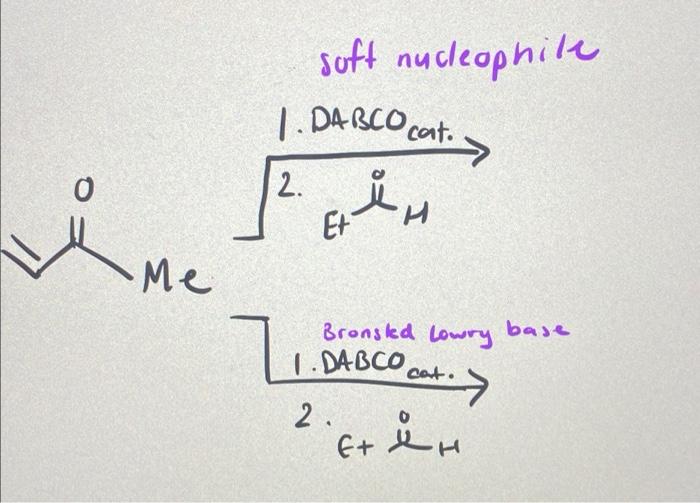
![SOLVED:The Baylis-Hillman reaction is a DABCO (1,4-diazabicyclo[2.2.2]octane) catalyzed reaction of an α, β-unsaturated carbonyl compound with an aldehyde to form an allylic alcohol. Propose a mechanism for the reaction. Propose a mechanism SOLVED:The Baylis-Hillman reaction is a DABCO (1,4-diazabicyclo[2.2.2]octane) catalyzed reaction of an α, β-unsaturated carbonyl compound with an aldehyde to form an allylic alcohol. Propose a mechanism for the reaction. Propose a mechanism](https://cdn.numerade.com/previews/fdf0f81c-0805-40e3-82ac-9584da897842_large.jpg)
SOLVED:The Baylis-Hillman reaction is a DABCO (1,4-diazabicyclo[2.2.2]octane) catalyzed reaction of an α, β-unsaturated carbonyl compound with an aldehyde to form an allylic alcohol. Propose a mechanism for the reaction. Propose a mechanism

The versatility of DABCO: synthetic applications of its basic, nucleophilic, and catalytic properties Part 1. Catalysis of Morita–Baylis–Hillman and Knoevenagel reactions | SpringerLink
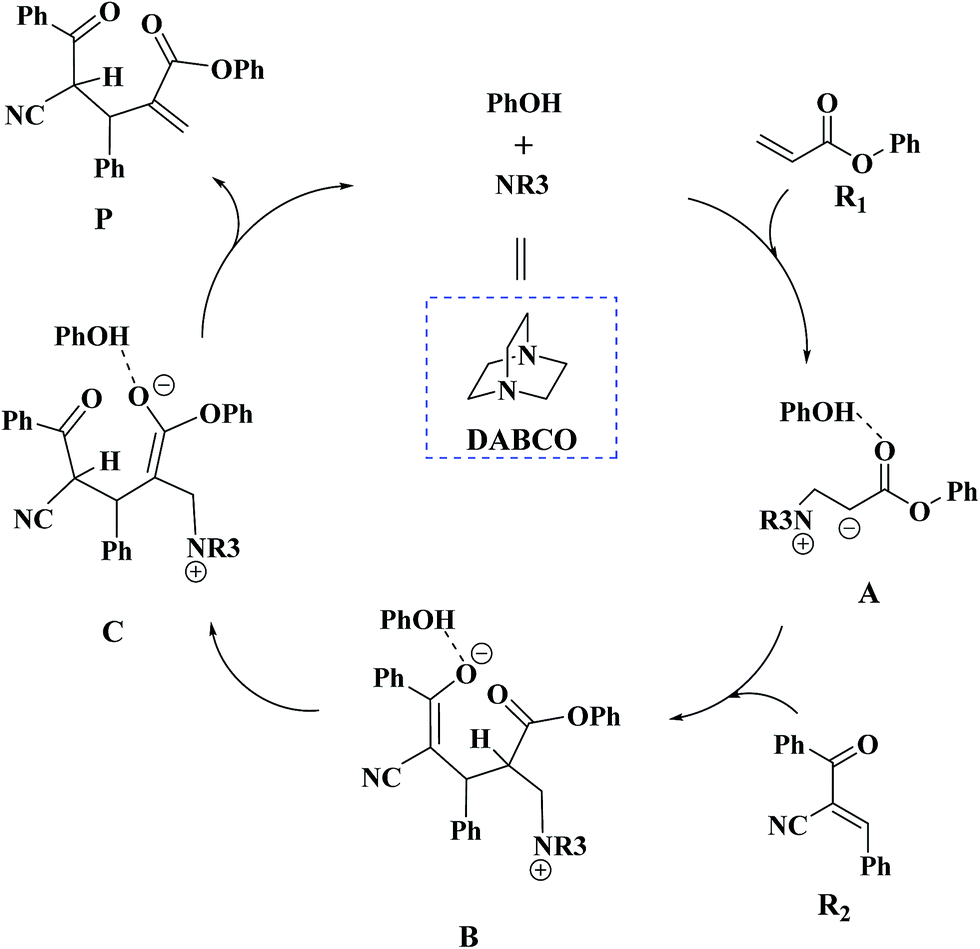
Mechanisms and stereoselectivities of the DABCO -catalyzed Rauhut–Currier reaction of α,β-unsaturated ketones and aryl acrylates: a computational inve ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA25311C
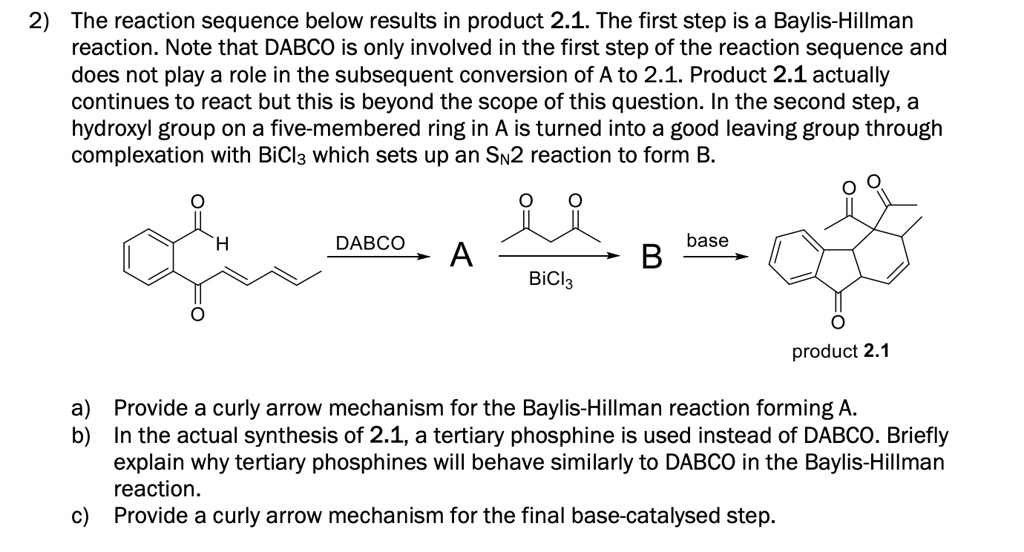
SOLVED: 2) The reaction sequence below results in product 2.1 The first step is a Baylis-Hillman reaction. Note that DABCO is only involved in the first step of the reaction sequence and

The versatility of DABCO: synthetic applications of its basic, nucleophilic, and catalytic properties Part 1. Catalysis of Morita–Baylis–Hillman and Knoevenagel reactions | SpringerLink
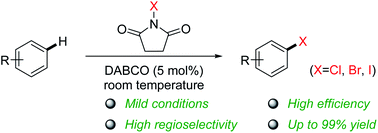
DABCO as a practical catalyst for aromatic halogenation with N-halosuccinimides - RSC Advances (RSC Publishing)
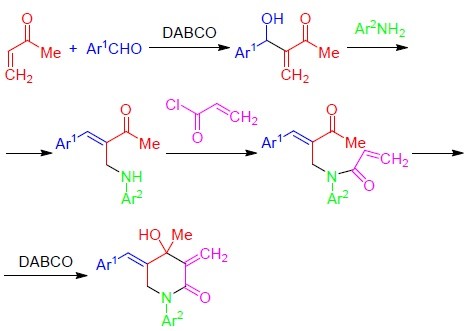
The versatility of DABCO: synthetic applications of its basic, nucleophilic, and catalytic properties Part 1. Catalysis of Morita–Baylis–Hillman and Knoevenagel reactions | SpringerLink

Nucleophilic Organic Base DABCO-Mediated Chemospecific Meinwald Rearrangement of Terminal Epoxides into Methyl Ketones | The Journal of Organic Chemistry
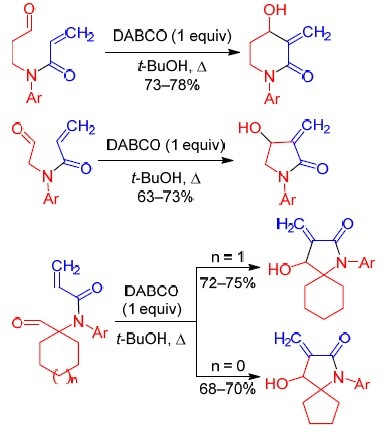
The versatility of DABCO: synthetic applications of its basic, nucleophilic, and catalytic properties Part 1. Catalysis of Morita–Baylis–Hillman and Knoevenagel reactions | SpringerLink

Dual Nucleophilic Catalysis with DABCO for the N-Methylation of Indoles | The Journal of Organic Chemistry

DABCO‐Catalysed Amidation under Assistance of Aerial Oxidation: Access to α‐ketoamides - Monga - 2018 - ChemistrySelect - Wiley Online Library