
Reactions of fluoronitrobenzenes with MTBD strong base in acetonitrile in the presence of water molecules - ScienceDirect

Representative examples of isolated and well-characterized nitrogen... | Download Scientific Diagram
![1,5,7‐Triazabicyclo[4.4.0]dec‐5‐ene Enhances Activity of Peroxide Intermediates in Phosphine‐Free α‐Hydroxylation of Ketones - Wang - 2021 - Angewandte Chemie - Wiley Online Library 1,5,7‐Triazabicyclo[4.4.0]dec‐5‐ene Enhances Activity of Peroxide Intermediates in Phosphine‐Free α‐Hydroxylation of Ketones - Wang - 2021 - Angewandte Chemie - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/6fc8955b-14e2-45e0-9718-323025818aed/ange202014478-fig-0001-m.jpg)
1,5,7‐Triazabicyclo[4.4.0]dec‐5‐ene Enhances Activity of Peroxide Intermediates in Phosphine‐Free α‐Hydroxylation of Ketones - Wang - 2021 - Angewandte Chemie - Wiley Online Library

Reactions of fluoronitrobenzenes with MTBD strong base in acetonitrile in the presence of water molecules - ScienceDirect
![1,5,7-Triazabicyclo[4.4.0]dec-1-ene (TBD), 7-methyl-TBD (MTBD) and the polymer-supported TBD (P-TBD): three efficient catalysts for the nitroaldol (Henry) reaction and for the addition of dialkyl phosphites to unsaturated systems - ScienceDirect 1,5,7-Triazabicyclo[4.4.0]dec-1-ene (TBD), 7-methyl-TBD (MTBD) and the polymer-supported TBD (P-TBD): three efficient catalysts for the nitroaldol (Henry) reaction and for the addition of dialkyl phosphites to unsaturated systems - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0040403999023400-fx1.gif)
1,5,7-Triazabicyclo[4.4.0]dec-1-ene (TBD), 7-methyl-TBD (MTBD) and the polymer-supported TBD (P-TBD): three efficient catalysts for the nitroaldol (Henry) reaction and for the addition of dialkyl phosphites to unsaturated systems - ScienceDirect
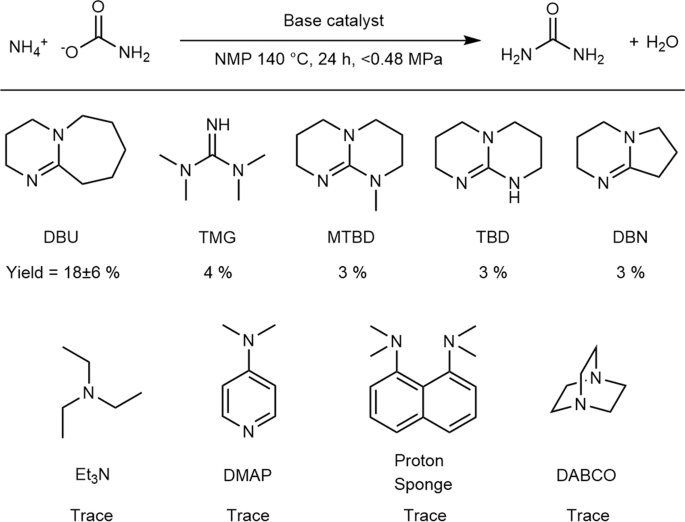
Organic bases catalyze the synthesis of urea from ammonium salts derived from recovered environmental ammonia | Scientific Reports

Molecules | Free Full-Text | An Investigation of the Organoborane/Lewis Base Pairs on the Copolymerization of Propylene Oxide with Succinic Anhydride

The Quest for the Ideal Base: Rational Design of a Nickel Precatalyst Enables Mild, Homogeneous C–N Cross-Coupling | Catalysis | ChemRxiv | Cambridge Open Engage
![Scheme 4. Hypothesized 7-Methyl-1,5,7-triazabicyclo [4.4.0]dec-5-ene... | Download Scientific Diagram Scheme 4. Hypothesized 7-Methyl-1,5,7-triazabicyclo [4.4.0]dec-5-ene... | Download Scientific Diagram](https://www.researchgate.net/publication/347740811/figure/fig3/AS:971949568176128@1608742243301/Scheme-4-Hypothesized-7-Methyl-1-5-7-triazabicyclo-440dec-5-ene-MTBD-promoted-CO2.png)
Scheme 4. Hypothesized 7-Methyl-1,5,7-triazabicyclo [4.4.0]dec-5-ene... | Download Scientific Diagram
![Inorganics | Free Full-Text | Temperature-Dependent Enhancement Effects for TBD (1,5,7-Triazabicyclo[4.4.0]dec-5-ene) with 2-Methylimidazole-Intercalated α-Zirconium Phosphate as a Latent Thermal Initiator in the Reaction of Glycidyl Phenyl Ether Inorganics | Free Full-Text | Temperature-Dependent Enhancement Effects for TBD (1,5,7-Triazabicyclo[4.4.0]dec-5-ene) with 2-Methylimidazole-Intercalated α-Zirconium Phosphate as a Latent Thermal Initiator in the Reaction of Glycidyl Phenyl Ether](https://www.mdpi.com/inorganics/inorganics-07-00083/article_deploy/html/images/inorganics-07-00083-sch001-550.jpg)



![Physical Properties of 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) | SpringerLink Physical Properties of 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) | SpringerLink](https://media.springernature.com/lw685/springer-static/image/art%3A10.1007%2Fs10765-019-2540-2/MediaObjects/10765_2019_2540_Fig2_HTML.png)

![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 95.0 %, TCI America, Quantity: 1 g | Fisher Scientific 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 95.0 %, TCI America, Quantity: 1 g | Fisher Scientific](https://assets.fishersci.com/TFS-Assets/CCG/Chemical-Structures/chemical-structure-cas-84030-20-6.jpg-650.jpg)

![Physical Properties of 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) | SpringerLink Physical Properties of 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) | SpringerLink](https://media.springernature.com/lw685/springer-static/image/art%3A10.1007%2Fs10765-019-2540-2/MediaObjects/10765_2019_2540_Fig7_HTML.png)



![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) Thermal Properties 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) Thermal Properties](https://thermtest.se/wp-content/uploads/2019/10/backbone-structure-dbu-tbd-mtbd.jpg)

![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) Thermal Properties 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) Thermal Properties](https://thermtest.se/wp-content/uploads/2019/10/7-Methyl-157-triazabicyclo4.4.0dec-5-ene-1.jpg)
![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene](https://www.sigmaaldrich.com/deepweb/content/dam/sigma-aldrich/structure5/151/mfcd00043004.eps/_jcr_content/renditions/mfcd00043004-large.png)