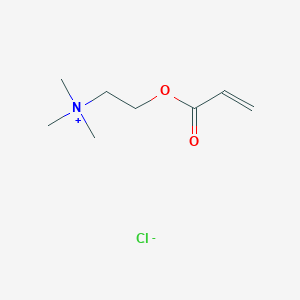
(2-Chloroethyl)trimethylammonium chloride, 98% (dry wt.), may cont. up to 5% water, Thermo Scientific Chemicals

Polymers | Free Full-Text | Study on Hydrolysis Properties and Mechanism of Poly(3-Methacrylamido Propyl Trimethyl Ammonium Chloride) Solution

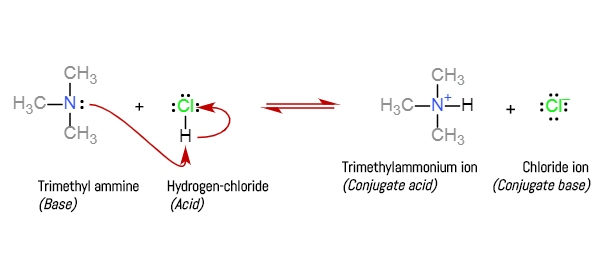
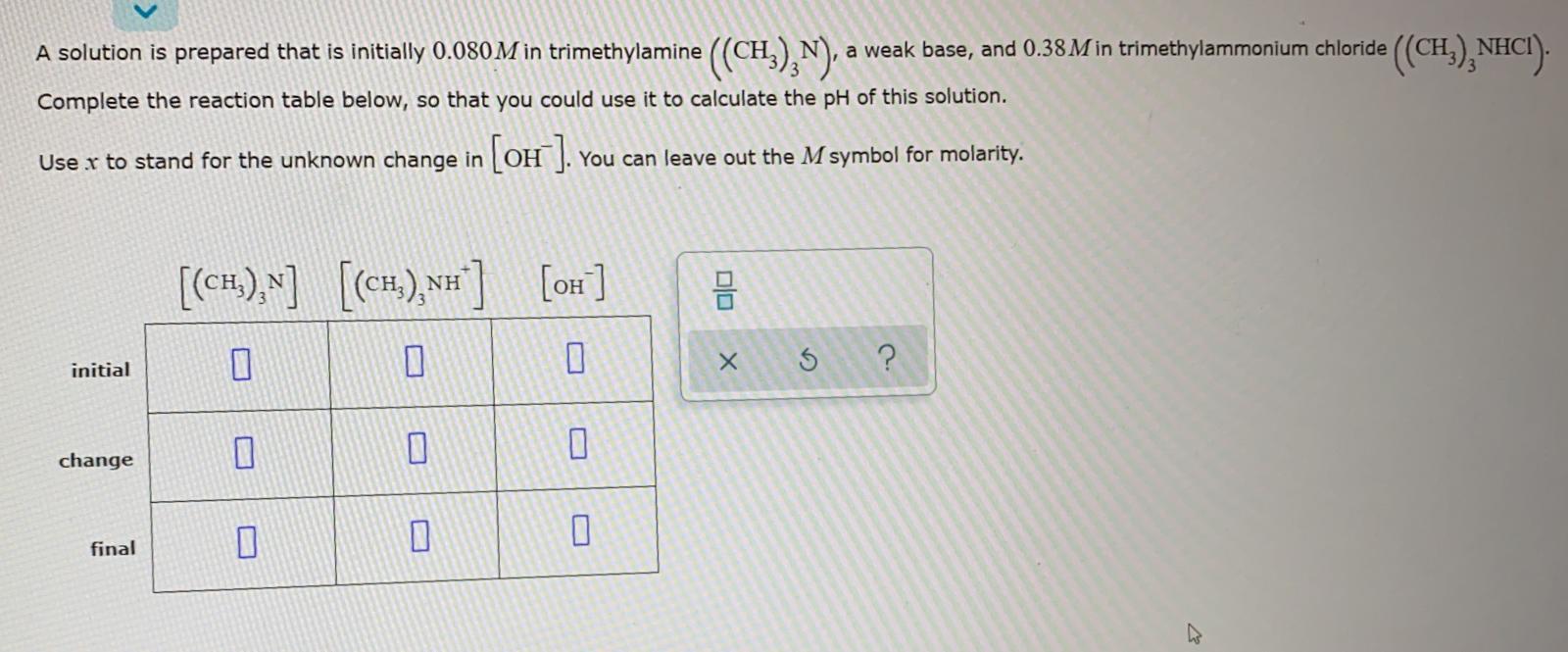
SOLVED: Determine the pH of a buffer which is a 0.20 M solution of trimethylamine (N(CH3)3) and a 0.40 M solution of trimethylammonium chloride (NH(CH3)3Cl). The Kb of trimethylamine at 25°C is
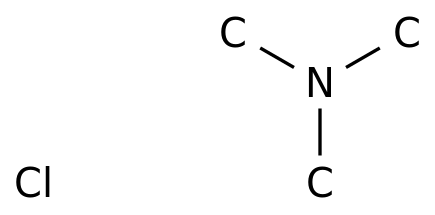
Carboxylic acid higher alcohol ester-trimethyl ammonium chloride and application thereof in clay stabilizer - Eureka | Patsnap develop intelligence library
![SOLVED: The trimethylammonium ion, [(CH3)3NH]+, is a weak acid. The acid dissociation equation is shown as: [CH3)3NH]+(aq) + H2O(l) ⇌ [H3O]+(aq) + (CH3)3N(aq) Ka = 1.55 x 10^-10 At 20°C, a saturated SOLVED: The trimethylammonium ion, [(CH3)3NH]+, is a weak acid. The acid dissociation equation is shown as: [CH3)3NH]+(aq) + H2O(l) ⇌ [H3O]+(aq) + (CH3)3N(aq) Ka = 1.55 x 10^-10 At 20°C, a saturated](https://cdn.numerade.com/ask_images/b2567ae091254414b640c7f5d6a0eff5.jpg)
SOLVED: The trimethylammonium ion, [(CH3)3NH]+, is a weak acid. The acid dissociation equation is shown as: [CH3)3NH]+(aq) + H2O(l) ⇌ [H3O]+(aq) + (CH3)3N(aq) Ka = 1.55 x 10^-10 At 20°C, a saturated
![Removal of dye from aqueous medium with pH-sensitive poly[(2-(acryloyloxy)ethyl]trimethylammonium chloride-co-1-vinyl-2-pyrrolidone] cationic hydrogel - ScienceDirect Removal of dye from aqueous medium with pH-sensitive poly[(2-(acryloyloxy)ethyl]trimethylammonium chloride-co-1-vinyl-2-pyrrolidone] cationic hydrogel - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S2213343720307855-ga1.jpg)
Removal of dye from aqueous medium with pH-sensitive poly[(2-(acryloyloxy)ethyl]trimethylammonium chloride-co-1-vinyl-2-pyrrolidone] cationic hydrogel - ScienceDirect
![Polymers | Free Full-Text | Hydrogels Based on Poly([2-(acryloxy)ethyl] Trimethylammonium Chloride) and Nanocellulose Applied to Remove Methyl Orange Dye from Water Polymers | Free Full-Text | Hydrogels Based on Poly([2-(acryloxy)ethyl] Trimethylammonium Chloride) and Nanocellulose Applied to Remove Methyl Orange Dye from Water](https://www.mdpi.com/polymers/polymers-13-02265/article_deploy/html/images/polymers-13-02265-g001.png)

![What Is [2-(Methacryloyloxy)ethyl]trimethylammonium chloride, Cas No 5039-78-1 Guide - ECHEMI What Is [2-(Methacryloyloxy)ethyl]trimethylammonium chloride, Cas No 5039-78-1 Guide - ECHEMI](https://file.echemi.com/fileManage/upload/canonicalSmiles/20220812/57dc97b3b0634b1f88e6c74e1b814fce.png)

![N-[3-(Trimethoxysilyl)propyl]-N,N,N-trimethylammonium chloride, 50% in methanol, N-[3-(Trimethoxysilyl)propyl]-N,N,N-trimethylammonium chloride, 50% in methanol,](https://www.thermofisher.com/TFS-Assets/CCG/Chemical-Structures/chemical-structure-cas-35141-36-7.jpg-250.jpg)